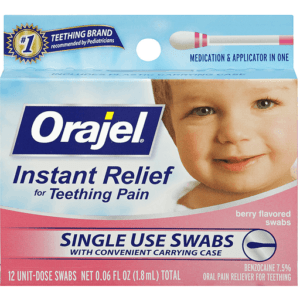
Based on communication from the U.S. Food and Drug Administration regarding the safety of over-the-counter (OTC) teething products containing Benzocaine, Orajel is discontinuing the distribution and sale of Orajel teething products containing Benzocaine
Reason for Recall: These products contain Benzocaine which may pose a serious health risk to infants and children. If you have the affected product, you may return it to your local store for a full refund.
Product(s) Affected:
UPC Description
| 31031003313 | ORAJEL BABY GEL .33 OZ |
| 31031033940 | ORAJEL BABY NITETIME .18 OZ |
| 31031034038 | ORAJEL BABY SWABS 12 CT |
Lot Code(s): All